Court Orders FDA To Release Pfizer Shot Data
FDA requests that court keeps info confidential for 55 years. Court responds by ordering FOIA compliance with the release of 500 documents per month.
On August 27, Public Health and Medical Professionals for Transparency (PHMPT) submitted a FOIA request to the FDA.
The FOIA request demanded that the FDA divulge “all data and information for the Pfizer Vaccine enumerated in 21 C.F.R. § 601.51(e) with the exception of publicly available reports on the Vaccine Adverse Events Reporting System” and requested that the process should be expedited.
The FDA attempted to deny the request for expedited processing claiming that the PHMPT had not demonstrated “a compelling need that involves an imminent threat to the life or physical safety of an individual.” The FDA issued the opinion that this request did not constitute an urgency to inform the public concerning actual or alleged federal government activity.
On September 16, PHMPT filed a lawsuit in the U.S. District Court of Northern Texas. The suit requested a court order to compel the FDA to comply with the FOIA request under law.
In response, the FDA proposed releasing 500 pages of Pfizer data per month. With a total of 329,000 pages of Pfizer data, according to the FDA’s statement in a joint report to the court, the FDA was asking for 55 years to release all documents.
PHMPT requested a 108-day deadline to release all documents. PHMPT said this would be “the same amount of time it took the FDA to review the responsive documents for the far more intricate task of licensing Pfizer’s Covid–19 vaccine.”
The judge has responded to PHMPT’s request by ordering the release of 500 documents per month.
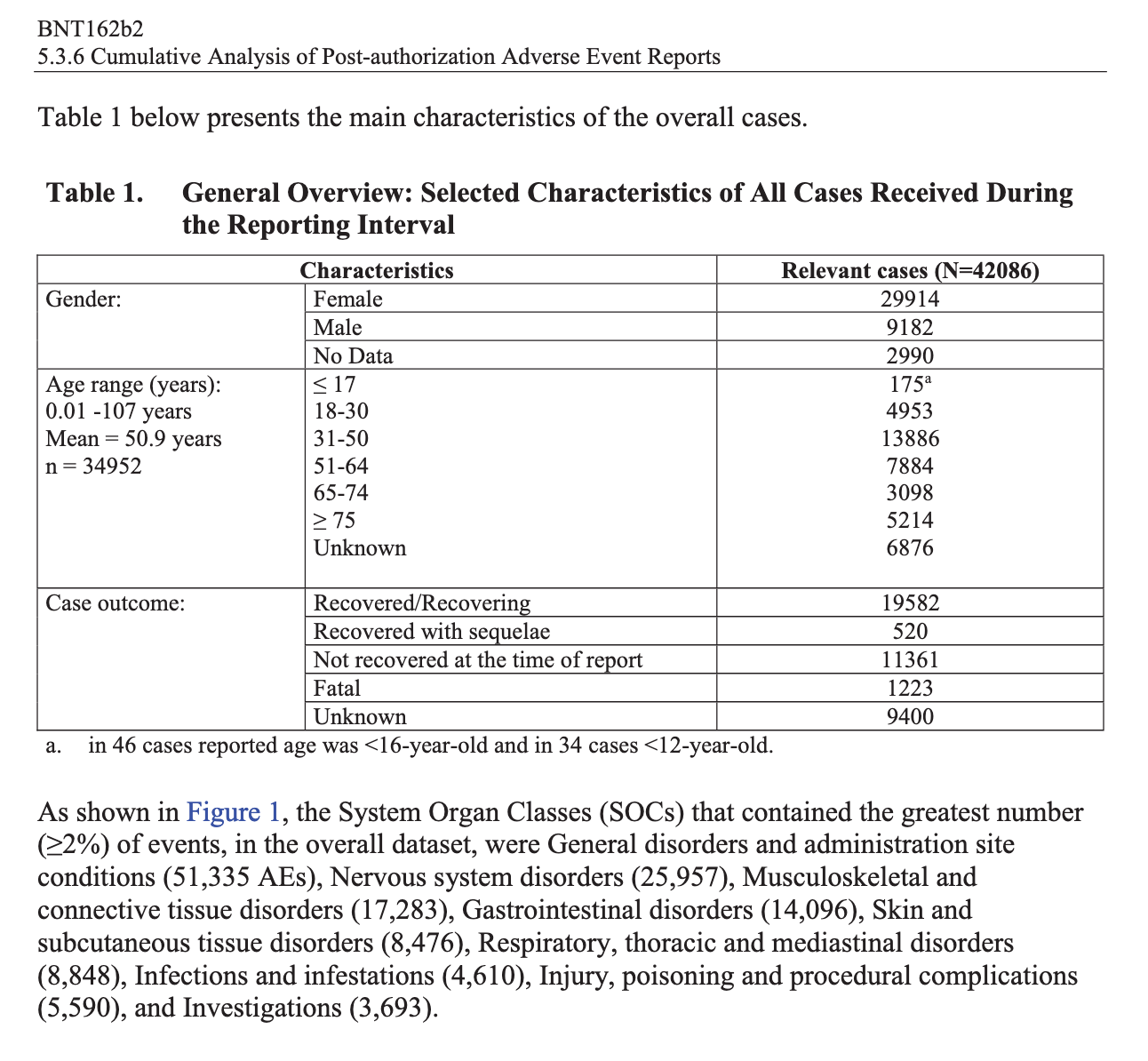
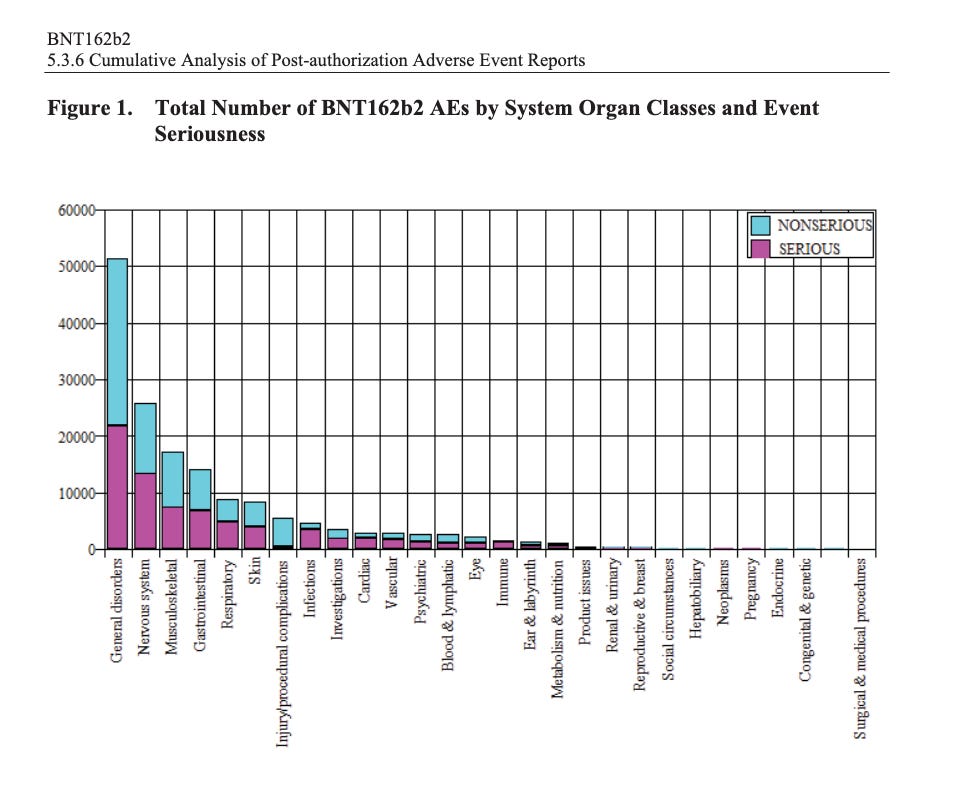
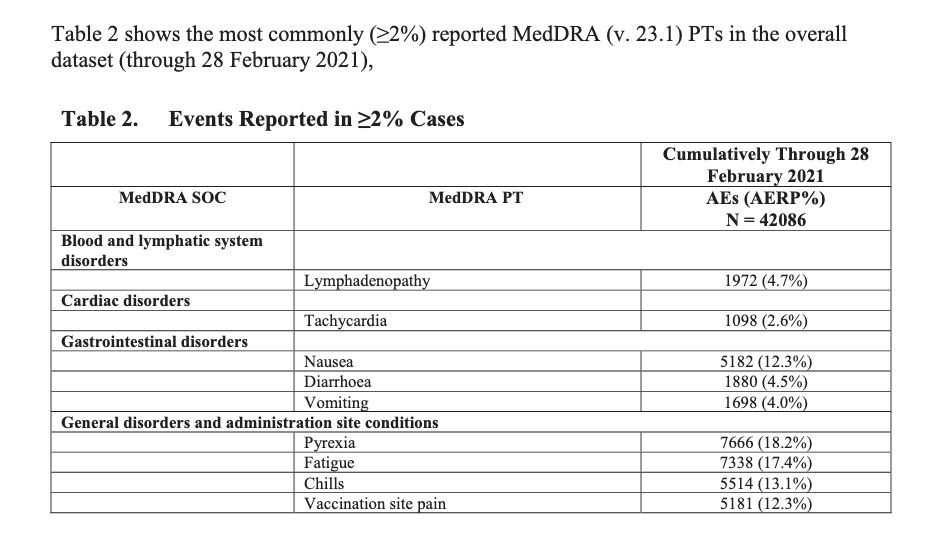
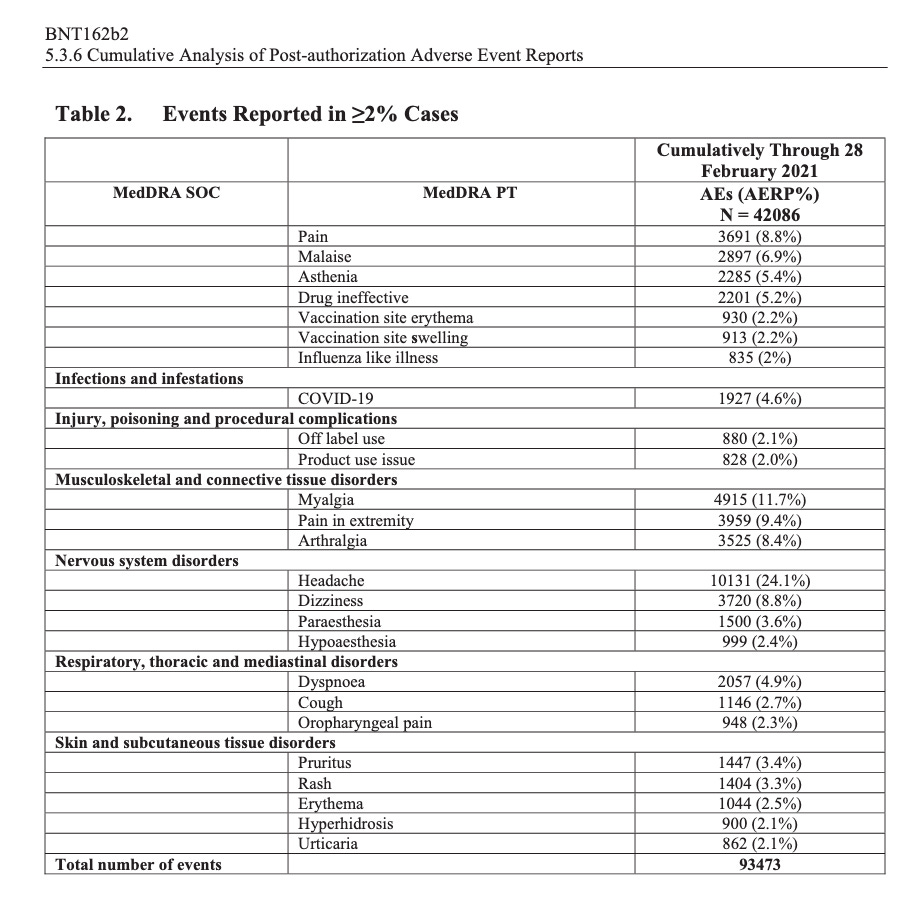
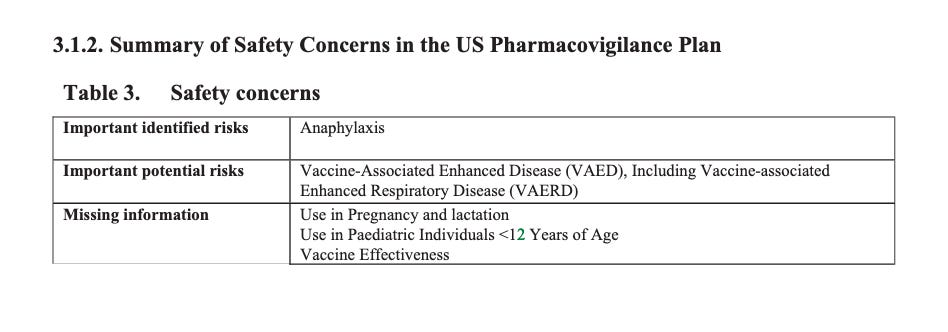
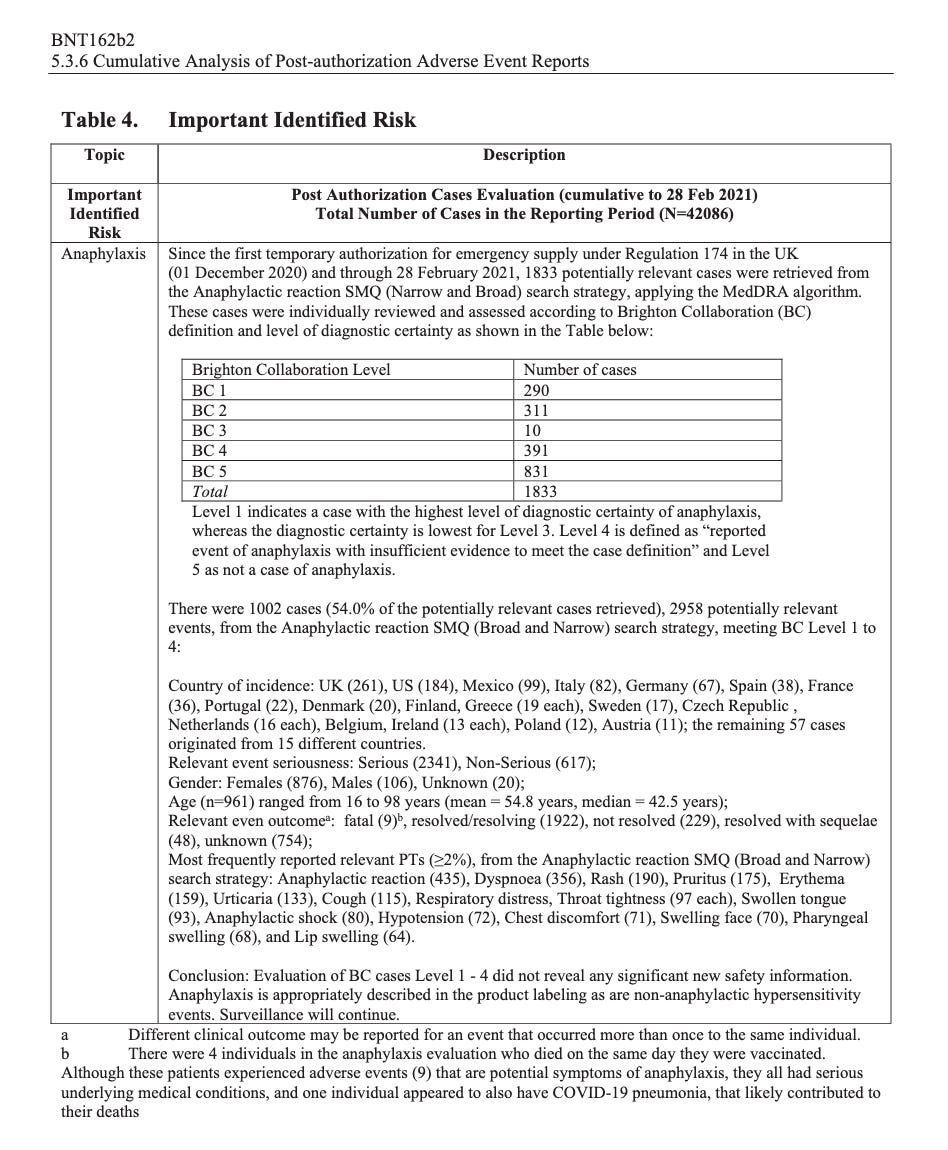
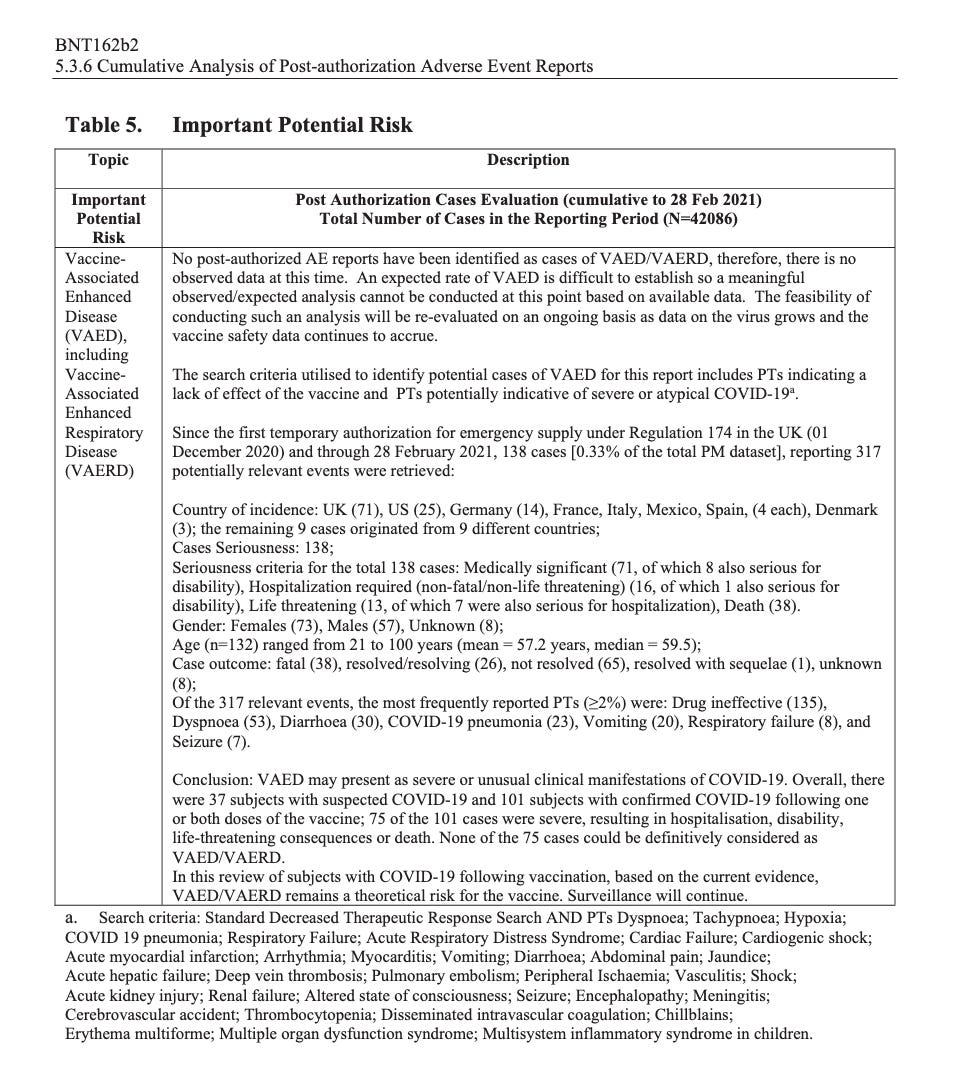
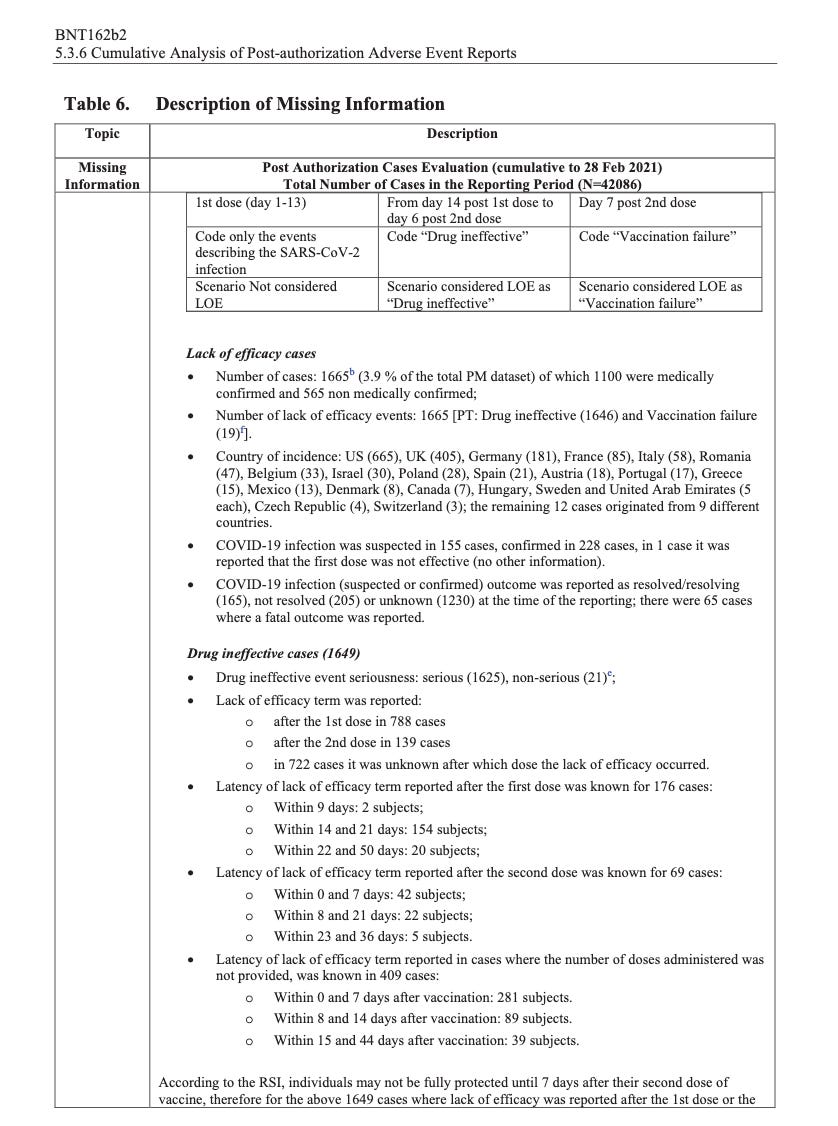
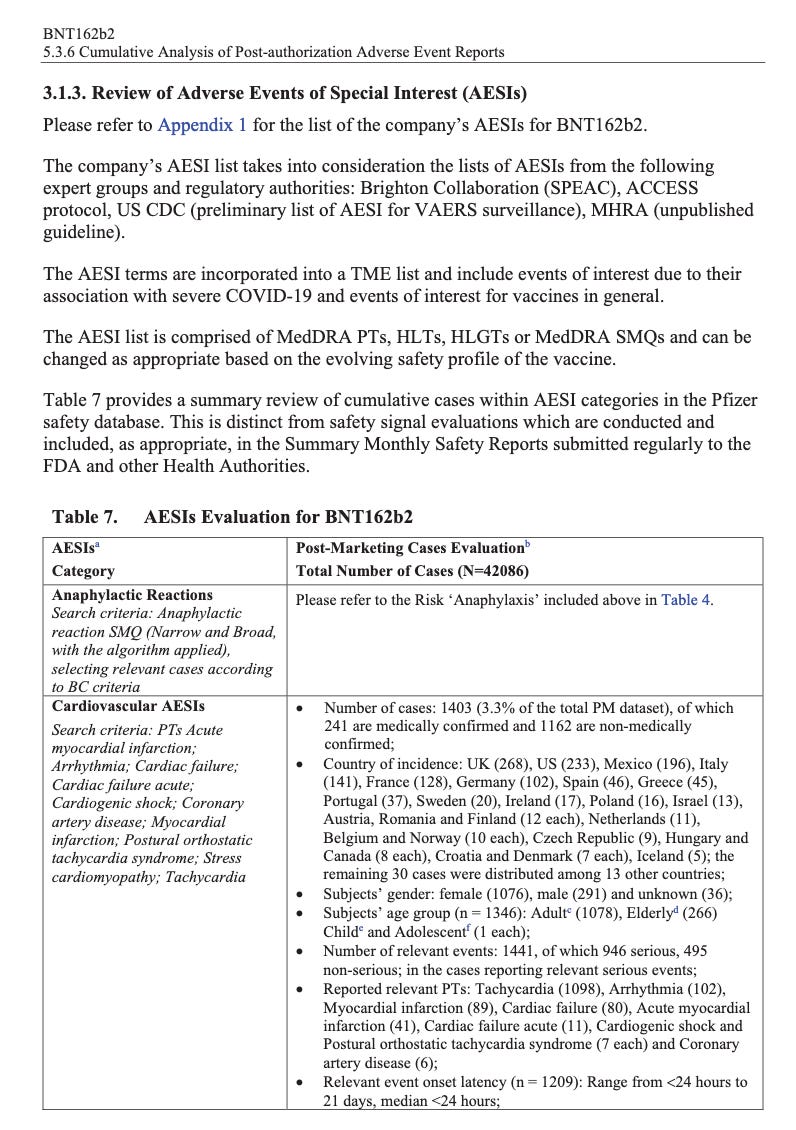
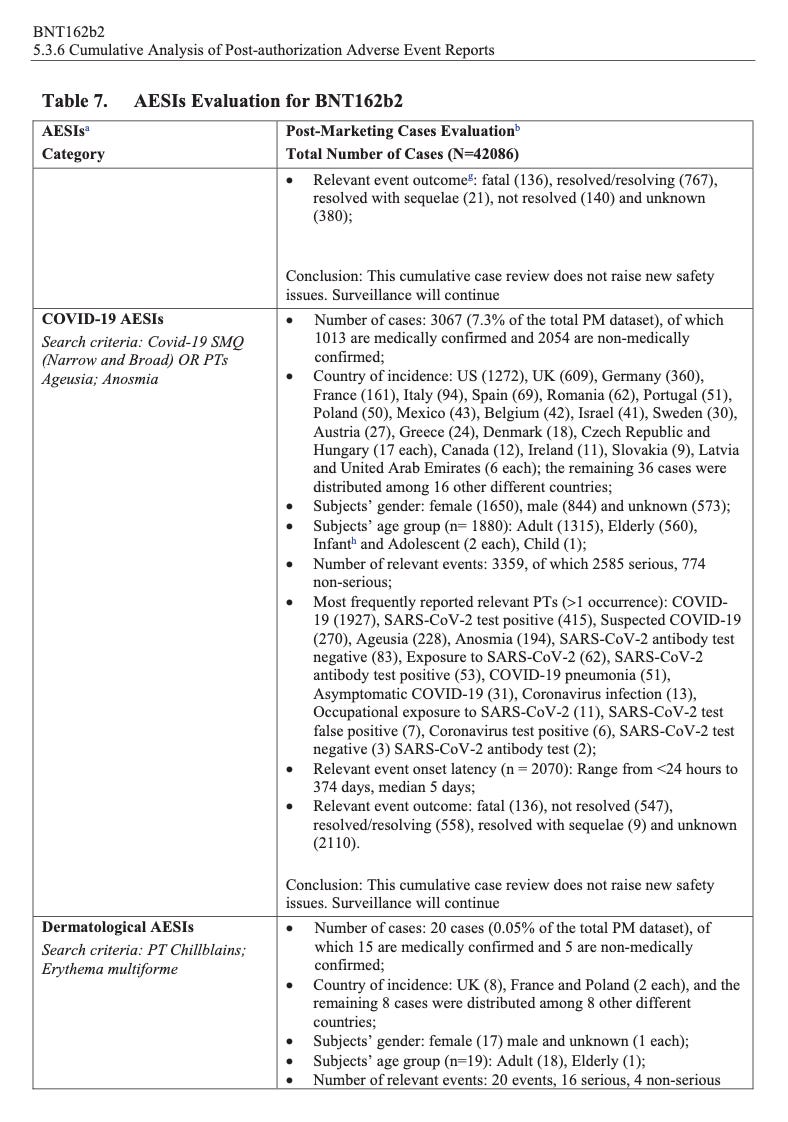
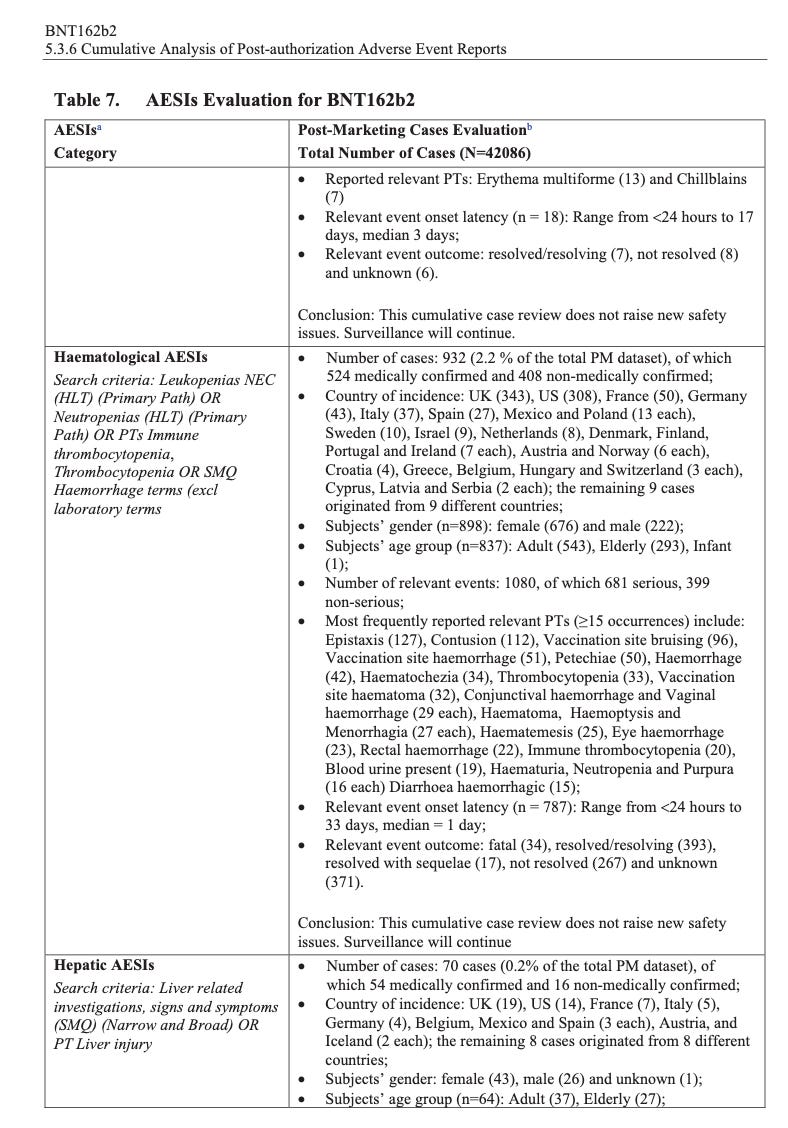
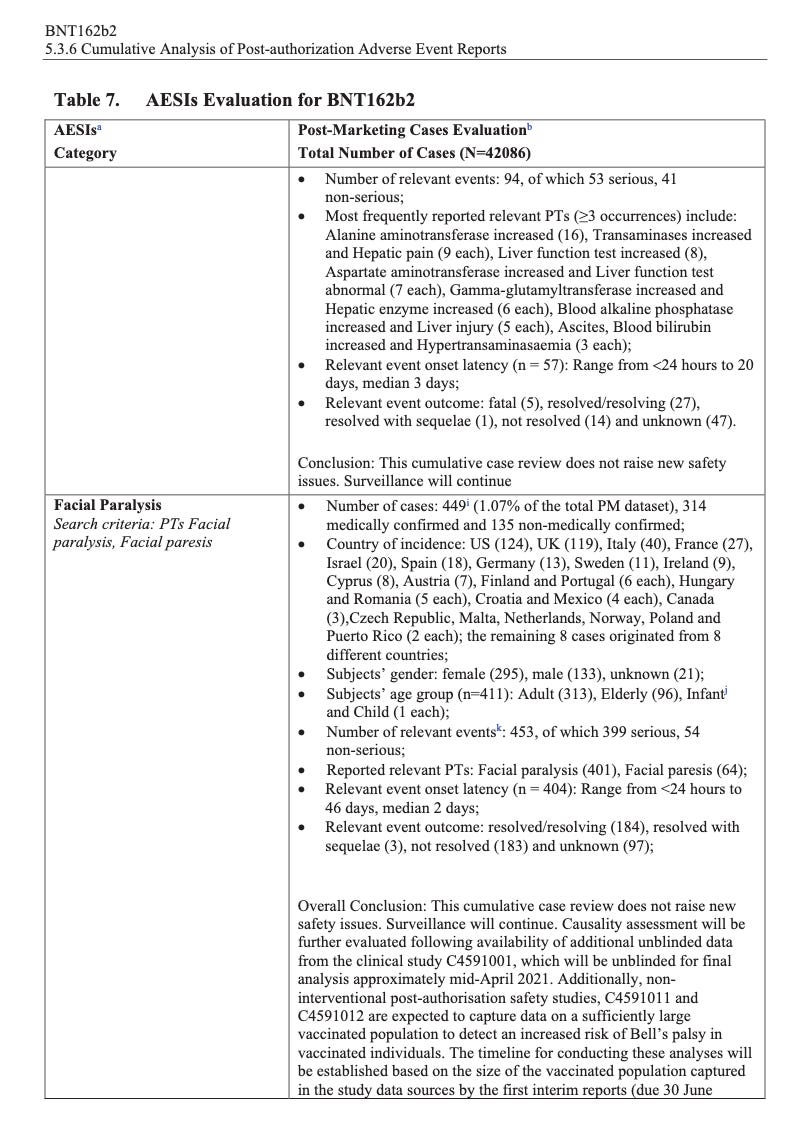
According to the first Pfizer and FDA documents released, 1,227 deaths were reported by February. These data were recorded in the first three months past the Pfizer rollout, December 1 to February 28. The FDA admits that they only recorded adverse events considered above mild. Many more reports of adverse events were never released.
Of adverse events reported from December 1 to February 28, one in every 37 adverse events was a death.
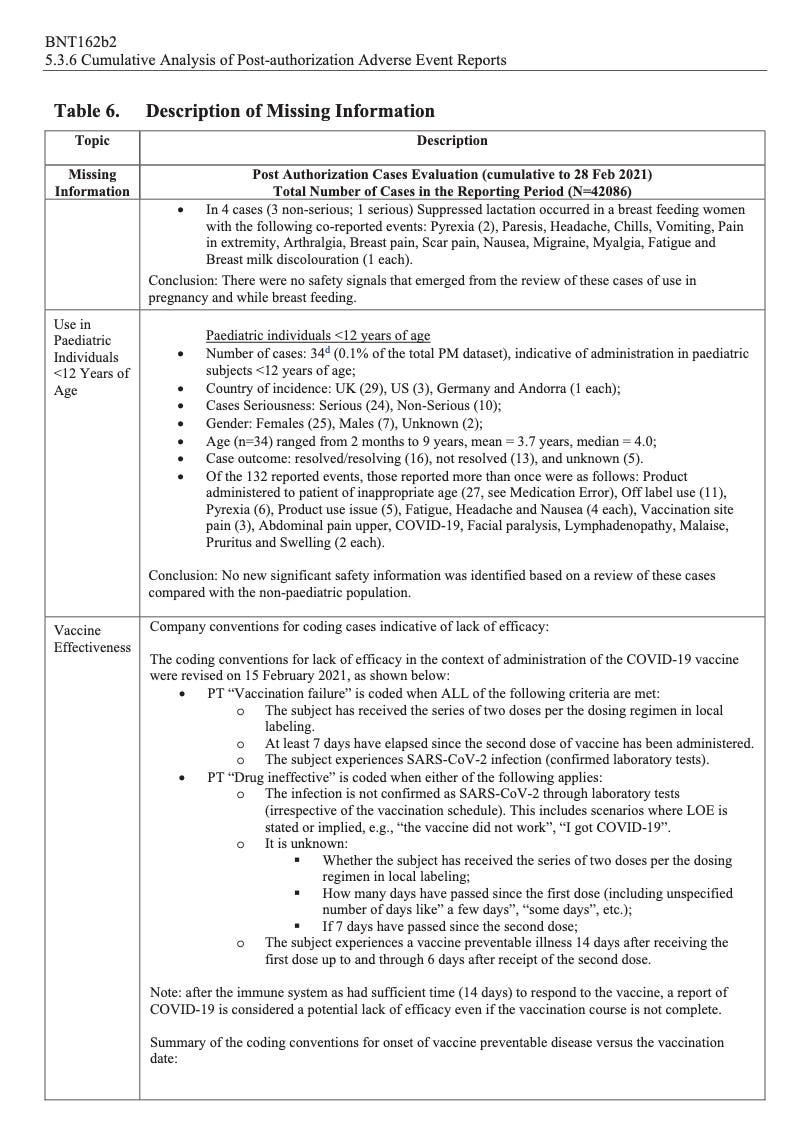
There were 270 cases reported cases of spontaneous abortion.
Seventeen serious cases involved breastfeeding babies. This is proof that the drug passes through lactating mothers.
See the rest of the document here: Adan_infowars. CUMULATIVE ANALYSIS OF POST-AUTHORIZATION ADVERSE EVENT REPORTS OF PF-07302048 (BNT162B2) RECEIVED THROUGH 28-FEB-2021. Reference is made to the Request for Comments and Advice submitted 04 February 2021 regarding Pfizer/BioNTech’s proposal for the clinical and post-authorization safety data package for the Biologics License Application (BLA) for our investigational COVID-19 Vaccine (BNT162b2). Further reference is made to the Agency’s 09 March 2021 response to this request, and specifically, the following request from the Agency. Scribd. [Note: Direct link to PDF provided by PHMPT is here.]
The Alex Jones Show. WATCH LIVE: THE MOST CENSORED NEWS BROADCAST IN THE WORLD. Banned.video. [Note: Video breaks down newly released Pfizer documents saying thousands of people were killed by the jab.]
Shalev, G. Thread. 1/ Bombshell. Twitter.